CABP Overview
A Broad Coverage Oral Monotherapy for CABP*1
* BAXDELA (delafloxacin) is indicated in adults for the treatment of community-acquired bacterial pneumonia (CABP) caused by the following susceptible microorganisms:
- Gram-positive organisms
- Staphylococcus aureus (methicillin-susceptible only)
- Streptococcus pneumoniae
- Gram-negative organisms
- Escherichia coli
- Klebsiella pneumoniae
- Haemophilus influenzae
- Haemophilus parainfluenzae
- Pseudomonas aeruginosa
- Atypical organisms
- Chlamydia pneumoniae
- Legionella pneumophila
- Mycoplasma pneumoniae
Safety Profile Demonstrated in Clinical Studies
- No evidence of QT prolongation was observed in patients taking BAXDELA 300 or 900 mg IV in a definitive QT study vs oral moxifloxacin 400 mg or placebo
- No clinically significant phototoxic potential was observed in BAXDELA 200 or 400 mg/day oral in a photosafety study vs lomefloxacin as the active comparator
- BAXDELA showed minimal potential for drug-drug interactions during development
- Does not inhibit cytochrome P450 at clinically relevant doses
- Oral BAXDELA should be taken 2 hours before or 6 hours after antacids or multivitamins containing iron or zinc.
-
Most Common Adverse Reactions
Occurring in ≥2% of Patients (from Phase 3 CABP trial)

Simple Oral Monotherapy
- No oral dosage adjustment required due to:
- 450-mg tablet BID for 5 to 10 days
- No food effect
BAXDELA is not recommended in patients with End Stage Renal Disease (ESRD) (eGFR >15 mL/min/1.73 m2), including patients on hemodialysis, due to insufficient information for dosing recommendations.
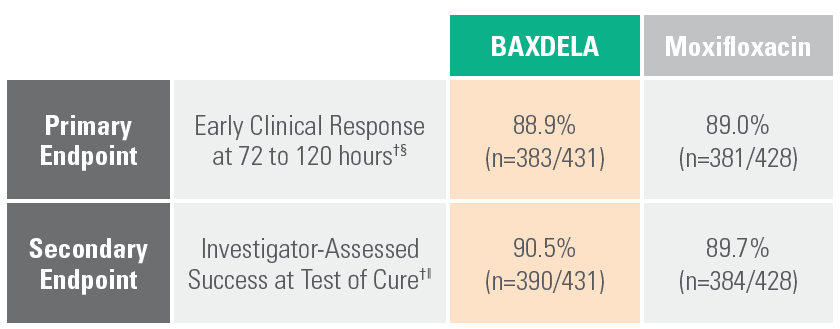
Demonstrated Efficacy Across Range of Patients with CABP*†‡1-3
BAXDELA was studied in a randomized, multicenter, multinational, double-blind, double-dummy, non-inferiority trial comparing BAXDELA 300 mg IV BID with an option to switch to BAXDELA tablet 450 mg PO BID to moxifloxacin 400 mg IV QD with an option to switch to moxifloxacin 400 mg PO QD.
BAXDELA Demonstrated Efficacy in Phase 3 trial vs. moxifloxacin
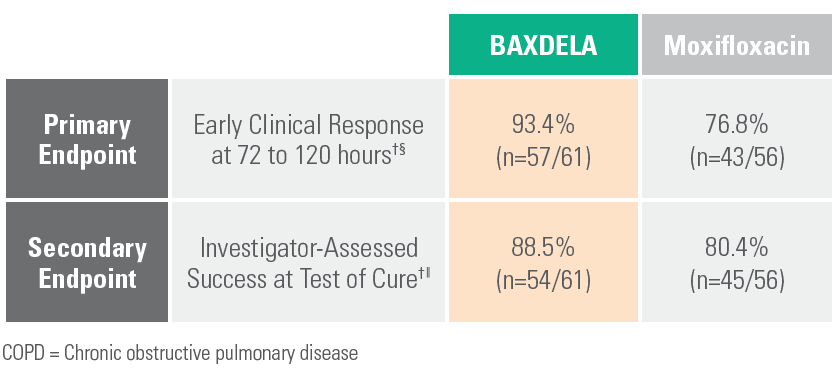
Pre-Specified Analysis in COPD/Asthma Subpopulation¶
* BAXDELA is indicated in adults for the treatment of
community-acquired bacterial pneumonia (CABP) caused by the
following susceptible microorganisms:
Streptococcus pneumoniae, Staphylococcus aureus
(methicillin-susceptible [MSSA] isolates only),
Klebsiella pneumoniae, Escherichia coli, Pseudomonas
aeruginosa, Haemophilus influenzae, Haemophilus
parainfluenzae, Chlamydia pneumoniae, Legionella
pneumophila,
and Mycoplasma pneumoniae.
To reduce the
development of drug-resistant bacteria and maintain the
effectiveness of BAXDELA and other antibacterial drugs, BAXDELA
should be used only to treat infections that are proven or
strongly suspected to be caused by susceptible bacteria.
† Evaluated in the intent to treat (ITT) population = all randomized patients.
‡ BAXDELA clinical trials demonstrated consistent efficacy across gender, age, weight, prior antibiotic use, baseline bacteremia, and PORT score in the CABP clinical trial.
§ Early Clinical Response (ECR) at 72-120 hours after the first dose, was defined as survival with improvement in at least two of four symptoms (cough, sputum production, chest pain, dyspnea) from baseline without deterioration in any of these symptoms, and without use of additional antimicrobial therapy for treatment of the current CABP infection due to lack of efficacy.
‖ Clinical response was also assessed by the investigator at the test of cure (TOC) visit and defined as survival with resolution or near resolution of the symptoms of CABP present at study entry, and no use of additional antimicrobial therapy for the current CABP infection, and no new symptoms associated with the current CABP infection.
¶ Exploratory analysis of the efficacy outcomes in prespecified subgroups.
1. BAXDELA (delafloxacin) [prescribing information].
2. Horcajada J, et al. A Phase 3 Study to Compare Delafloxacin With Moxifloxacin for the Treatment of Adults With Community-Acquired Bacterial Pneumonia (DEFINE-CABP). Open Forum Infec Dis 2019. https://doi.org/10.1093/ofid/ofz514
3. Data on File, Melinta Therapeutics, LLC.
INDICATION & USAGE:
BAXDELA is indicated in adults for the treatment of acute bacterial skin and skin structure infections (ABSSSI) caused by the following susceptible microorganisms: Staphylococcus aureus (including methicillin-resistant [MRSA] and methicillin-susceptible [MSSA] isolates), Staphylococcus haemolyticus, Staphylococcus lugdunensis, Streptococcus agalactiae, Streptococcus anginosus group (including Streptococcus anginosus, Streptococcus intermedius, and Streptococcus constellatus), Streptococcus pyogenes, and Enterococcus faecalis, Escherichia coli, Enterobacter cloacae, Klebsiella pneumoniae, and Pseudomonas aeruginosa.
Melinta Therapeutics, LLC - Unparalleled in passion & purpose
Medical Information
For medical inquiries or to report an
adverse event, other safety-related information, or product
complaints for a company product, please contact Medical
Information.
Phone: 1-844-MED-MLNT (1-844-633-6568)
medinfo@melinta.com
www.melintamedicalinformation.com
©2021 All rights reserved. PP-BAX-US-0399 Terms of Use
